Immunotherapy has emerged as a promising approach to treating several forms of cancer. Use of immune cells, such as natural killer (NK) cells, along with small molecule drugs and antibodies through antibody dependent cell-mediated cytotoxicity (ADCC) has been investigated as a potential combination therapy for some difficult to treat solid tumors. Nevertheless, there remains a need to develop tools that support co-culture of target cancer cells and effector immune cells in a contextually relevant three-dimensional (3D) environment to provide a rapid means to screen for and optimize ADCC-drug combinations. To that end, we have developed a high throughput 330 micropillar-microwell sandwich platform that enables 3D co-culture of NK92-CD16 cells with pancreatic (MiaPaCa-2) and breast cancer cell lines (MCF7 and MDA-MB-231). The platform successfully mimicked hypoxic conditions found in a tumor microenvironment (Figure 1) and was used to demonstrate NK-cell mediated cell cytotoxicity in combination with two monoclonal antibodies; Trastuzumab and Atezolizumab (Figure 2). The platform was also used to show dose response behavior of target cancer cells with reduced EC50 values for paclitaxel (an anti-cancer chemotherapeutic) when treated with both NK cells and antibody. Such a platform may be used to develop more personalized cancer therapies using patient-derived cancer cells. [Gopal et al. Commun. Biol, 4, 893, 2020]. We are transitioning to organoid-on-chip cultures. Along these lines, various cell types are being investigated, including neural (neurons, astrocytes) and immune (macrophage and microglia). New results will be forthcoming.
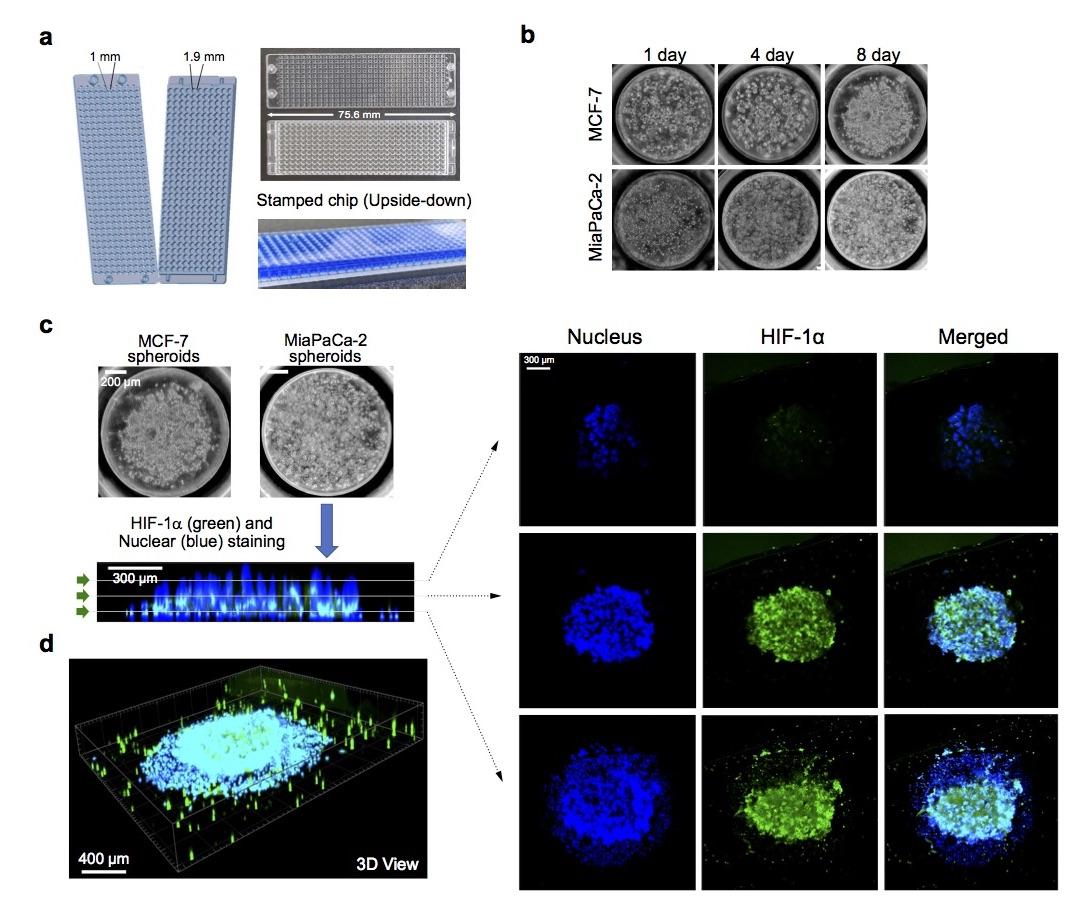
Figure 1. Generation of 3D tumor spheroid micropillar array. (a) Photo of 330-micropillar/well chip for effector cell-mediated cytotoxicity. Diameters of micropillar and microwell array spots are 1 and 1.9 mm, respectively. (b) Generation of 3D tumor spheroid by printing high density cells (~2500 cells/250 nL) in Matrigel, followed by culturing cancer cells for up to 8 days. (c) Expression of HIF-1α inside MiaPaCa-2 3D tumor spheroids. Confocal microscopy images of MiaPaCa-2 3D tumor spheroids stained with Hoechst 33342 and labeled with HIF-1α antibody via immunofluorescence. Top views of cross-sectional images in a 3D tumor spheroid at different focal planes (top, middle, and bottom) using Imaris software. A green arrow symbol orients the viewing direction for the cross-sections reconstructed from Z stacks. (d) 3D view of a 3D tumor spheroid after staining nucleus and labeling HIF1α.
Current Collaborator:
Bosung Ku – MBD Korea, Ltd.
