As many essential elements of the immune system undergo circadian oscillations, a mechanism behind the relationship between circadian disruption (CD) and increased rates of Alzheimer’s disease and related dementias (ADRDs) could stem from dysregulation of circadian inflammatory responses. Microglia, the innate immune cells of the central nervous system, are involved in inflammation and immune responses and play a significant role in contributing to neuroinflammation associated with neurodegenerative diseases. In their resting state, microglia carry out continuous surveillance of the brain environment, sampling areas to maintain homeostasis. The ‘activated’ microglial state is itself an oversimplification and comprises multiple phenotypes, broadly differentiated into ‘pro-inflammatory’ and ‘anti-inflammatory’ states. The pro-inflammatory phenotype is associated with the release of mediators, which promote extermination of the stimulus, whereas the anti-inflammatory phenotype is associated with the release of mediators that promote wound healing, debris clearance and homeostasis. The disruption of macrophage/microglia function could be a mechanistic link between CD and ADRDs (Figure 1).
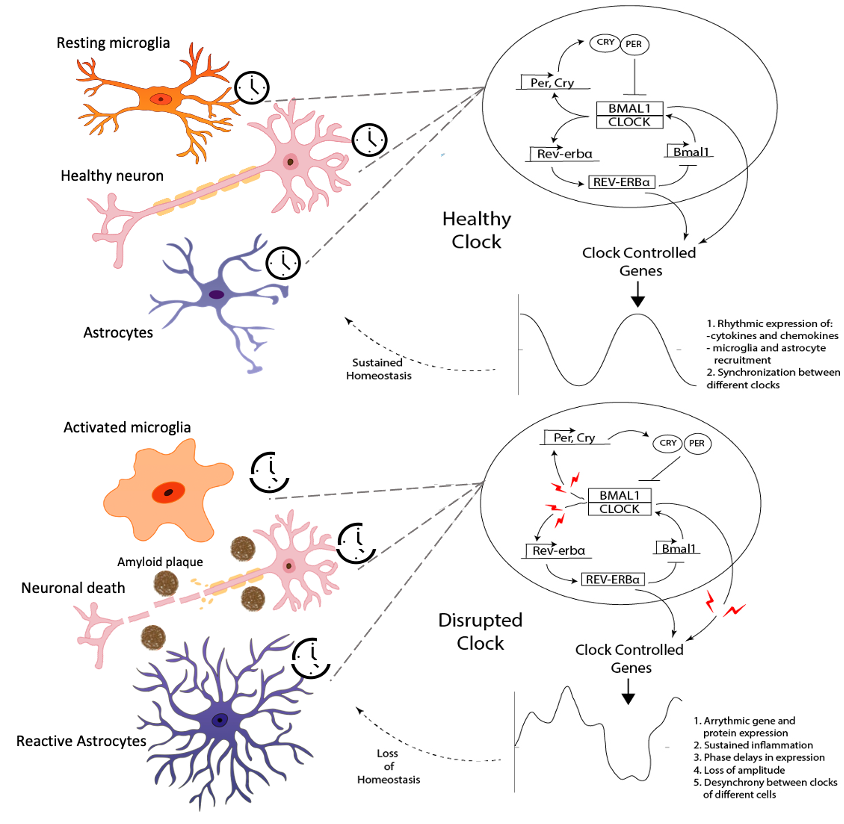
Figure 1. Interplay of circadian clock and neuroinflammation. A) Under normal homeostatic conditions, the clock in each cell is maintained by a feedback-based regulation in expression of clock genes. This results in rhythmicity in the expression of clock and clock-controlled genes. B) In case of neurodegenerative diseases, the presence of toxic aggregated proteins like Aβ (and likely tau) results in disruption of feedback between clock genes. This leads to loss of rhythmicity in expression of clock and clock-controlled genes that triggers sustained neuroinflammation and neuronal death.
Current Collaborators:
Jennifer Hurley – Rensselaer Polytechnic Institute
Mariana Figueiro – Icahn School of Medicine at Mount Sinai
We hypothesize that circadian rhythms play a pivotal role in the nature and progression of macrophage/microglial responses towards amyloid beta (Aβ), and that by using patient-derived macrophages and neurons, we can address how CD impacts human macrophages at the individual level. To test this set of hypotheses, we are developing a high-throughput, 3D microscale neuronal organoid-on-a-chip (NOC) platform to serve as the screening module for evaluating the effect of CD on neuroinflammation. Sustained neuroinflammation is a major contributor to the progression of neurodegenerative diseases such as Alzheimer’s (AD) and Parkinson’s (PD) diseases. Neuroinflammation, like other cellular processes, is affected by the circadian clock. Microglia, the resident immune cells in the brain, act as major contributors to neuroinflammation and are under the influence of the circadian clock. Microglial responses such as activation, recruitment, and cytokine expression are rhythmic in their response to various stimuli. While the link between circadian rhythms and neuroinflammation is clear, significant gaps remain in our understanding of this complex relationship. To further our understanding of this relationship, we studied the interaction between the microglial circadian clock and the enzyme NADPH Oxidase Isoform 2 (NOX2), an enzyme essential for the production of reactive oxygen species (ROS) in oxidative stress, an integral characteristic of neuroinflammation [Muthukumarasamy et al. Front. Immunol. 14, 1106515, 2023]. We examined BV2 microglia over circadian time, demonstrating oscillations of the clock genes Per2 and Bmal1 and the NOX2 subunits gp91phox and p47phox (Figure 2A and B) We discovered that the BV2 microglial clock exerted significant control over NOX2 expression and that the inhibition of NOX2 enabled the microglia to retain a functional circadian clock while reducing levels of ROS and inflammatory cytokines. These trends were mirrored in mouse bone marrow-derived primary macrophages (Figure 2C). In addition, NOX2 inhibition affected the levels of inflammation associated cytokines in BV2 microglia (Figure 2D). Our findings indicate NOX2 plays a crucial role in the interaction between the circadian clock and the activation of microglia/macrophages into their pro-inflammatory state.
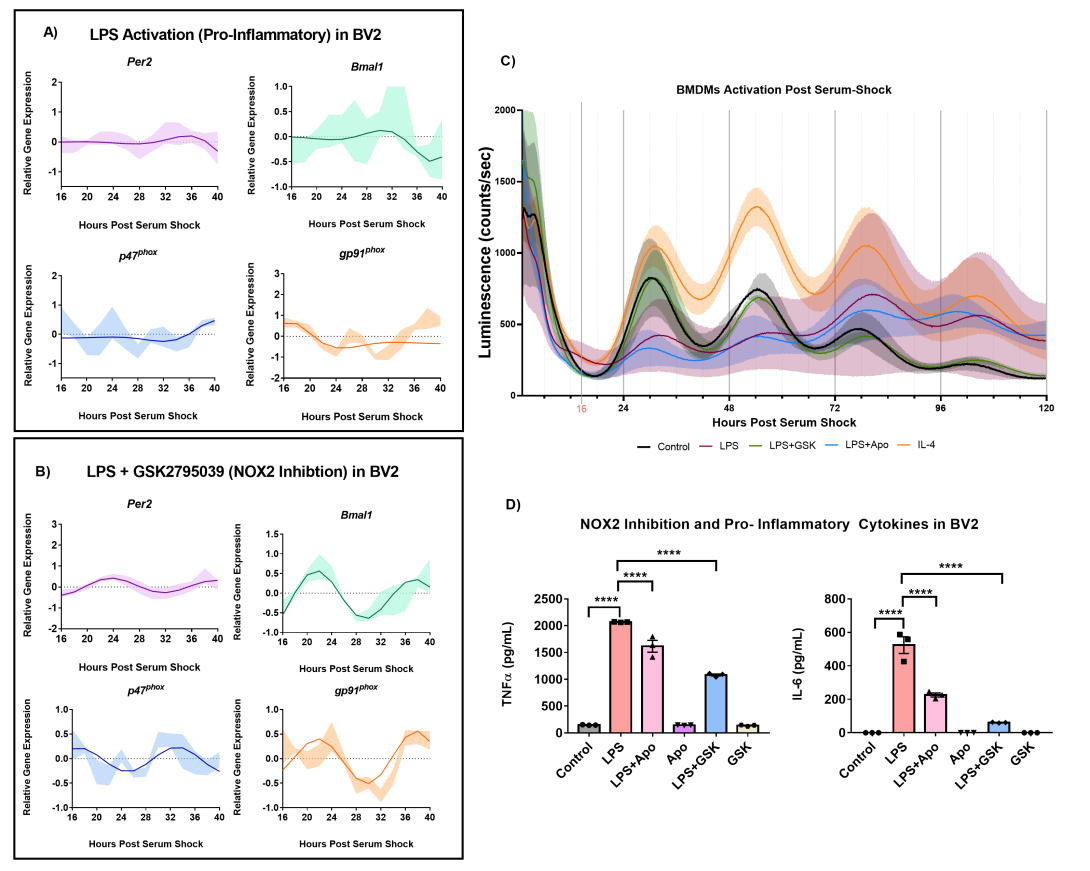
Figure 2. LPS (1 μg/mL) activation in BV2 microglia. (A) ECHO fitted plots for mRNA expression of clock genes (n = 3) Per2 and Bmal1. (B) ECHO fitted plots for mRNA expression of NOX2 components gp91phox and p47phox in BV2 microglia in the presence of 1 μg/mL LPS and 25 μM GSK27895039. Data represented as fold change in expression using Hprt1 as a reference gene and HSP0 as a reference sample for the ΔΔCt method of data analysis. Bold line represent model fit with shaded region representing the standard deviation of model at each time point. All plots had p<0.05 for ECHO significance fit. (C) Inhibition of NOX2 by GSK2795039 under LPS activation conserves PER2 oscillation in mouse bone marrow-derived macrophages (BMDMs). Luminescence traces obtained luciferase measurement of serum-shock synchronized mouse BMDMs with and without LPS, and NOX2 inhibitors. Cells were exposed to LPS with and without NOX2 inhibitors apocynin and GSK2795039 during the starve stage of the synchronization protocol. (Luciferase measurements were obtained by the LumiCycle HPS0 to HPS120 (n = 3). Data are represented as mean ± SEM. (D) NOX2 inhibition affects the levels of inflammation associated cytokines in mouse bone marrow-derived macrophages; TNF-α and IL-6 (pro-inflammatory cytokines). Data are represented as mean ± SEM and analyzed using single-factor ANOVA test. ** denotes p<0.01, *** denotes p<0.001 and **** denotes p<0.0001.
